
Structure of Batch Manufacturing Record YouTube
A batch manufacturing record or BMR is a very important document & it contains all the manufacturing history of the batch so always put the real-time & actual data in the BMR during each step & don't manipulate the data because the manipulated or fake data will create difficulties during the investigation of any batch if required.
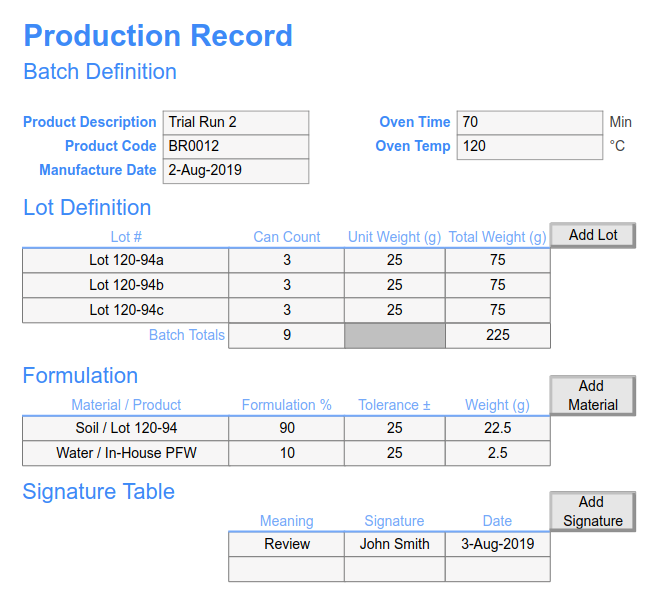
Batch Records SciCord, LLC
A master batch record (MBR), also known as a master production record (MPR) is a document that contains the approved ingredients, formulation, and instructions guiding the production of a pharmaceutical product. The document enables manufacturers to follow the necessary regulatory guidelines when manufacturing a product.

Batch records are a critical part of maintaining Good Manufacturing Practices. This excel
A batch manufacturing record is a written and validated narration of the end-to-end manufacturing process. It includes the records of production, instructions on how to manufacture a product, quality control measures, the procedures involved, inspection reports, and the report on the outcome.

How to Prepare a Batch Manufacturing Record & Free Template (2022)
A batch manufacturing record is a document designed to provide a complete record of the manufacturing history of a batch of product. The terminology is widely applied within the Pharmaceutical & Chemical industries and is referenced in many of the pharmaceutical and food regulatory agency requirements. The US Food and Drug administration.

Batch Manufacturing Record (BMR) and BPR
G. Batch Production Record Review (6.7) Written procedures should be established and followed for the review and approval of batch production and laboratory control records, including packaging.
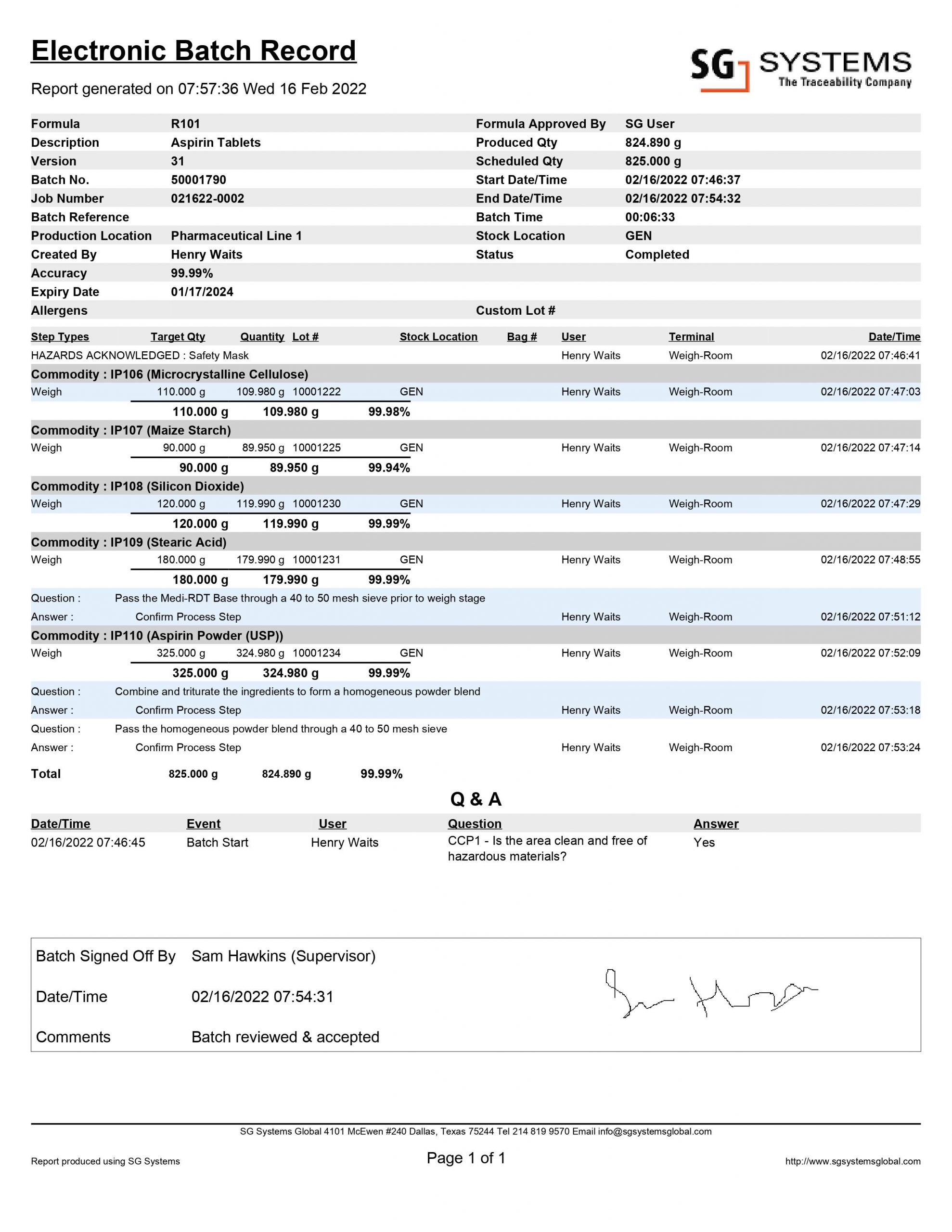
Electronic Batch Record (EBR) 21 CFR Part 11 Digital Compliance
Batch Manufacturing Records (BMR) are essential to any pharmaceutical or medical device manufacturing. These documents allow manufacturers to track and document the production process and ensure product quality. From start to finish, batch records provide an audit trail of the manufacturing process and the materials used.

Check List of Batch Record Production And Manufacturing Industrial Processes
What should be included in a batch manufacturing record? A Batch Manufacturing Record (BMR) is a document that contains step-by-step documentation of the entire manufacturing process involved in producing a product batch, including the expected batch yields and labeling requirements.

BMR Batch manufacturing record BMR Forms Pharma YouTube
A batch manufacturing record is a written document from the batch that is prepared during the pharmaceutical manufacturing process. It contains step-by-step batch production and actual information of the entire production process. There are several steps in the pharmaceutical product manufacturing process.

Sample of Batch Manufacturing Record (BMR) Atorvastatin PDF Download M A N O X B L O G
A batch manufacturing record, or BMR, is a document containing the details of the manufacture of each product batch, across the whole manufacturing process. As there are many stages in the manufacturing process, each step must be recorded as proof, from obtaining the raw materials through to the final stage of packaging ready for sale.
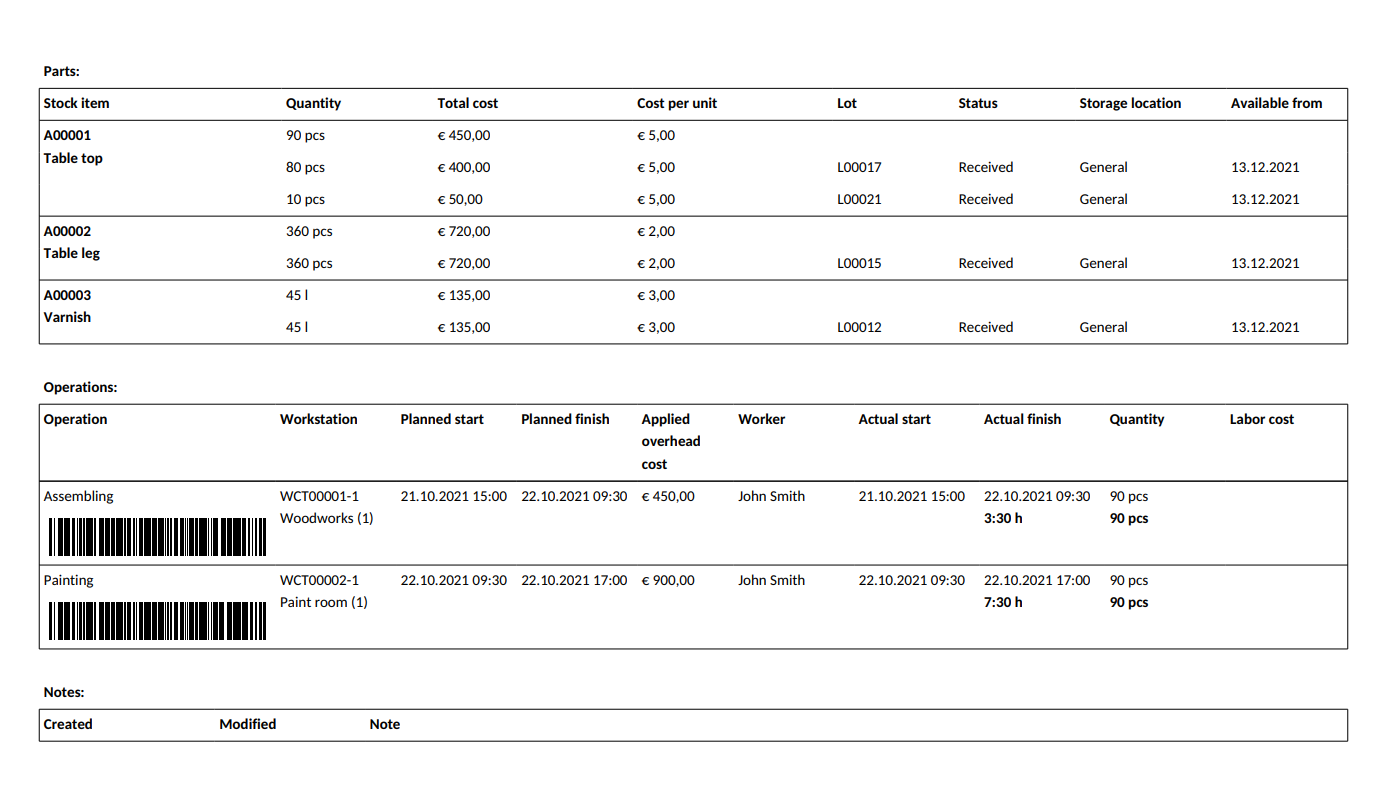
How to Keep Perfect Batch Records? MRPeasy
Batch manufacturing record is a written document from the batch that is prepared during the pharmaceutical manufacturing process. It contains actual data of the batch manufacturing and whole manufacturing process step by step. There are several stages of the pharmaceutical product manufacturing process.
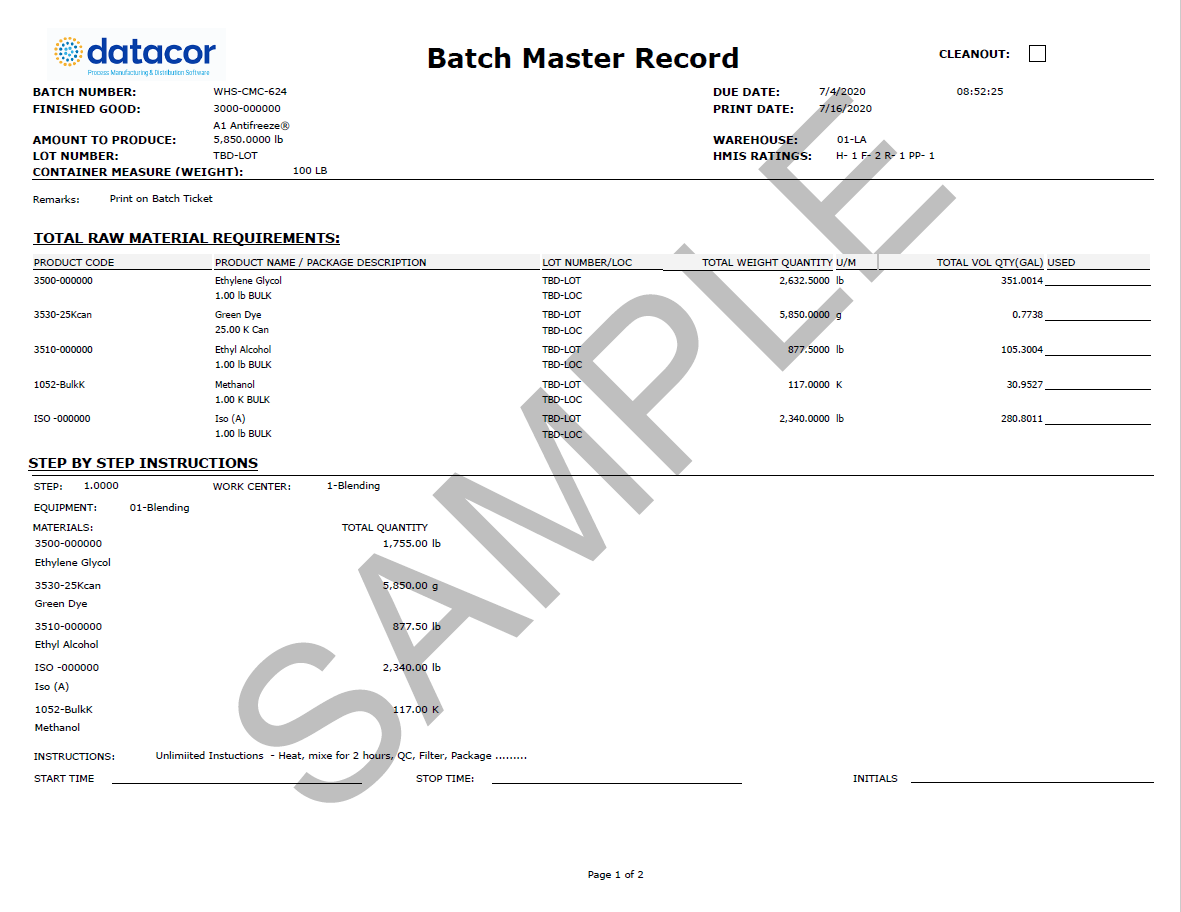
How to Prepare a Batch Manufacturing Record & Free Template (2023)
A batch manufacturing record (BMR) is an important document for chemical and process manufacturers: It tells users how to produce a batch of a given product, then records the entire production process, from start to finish.
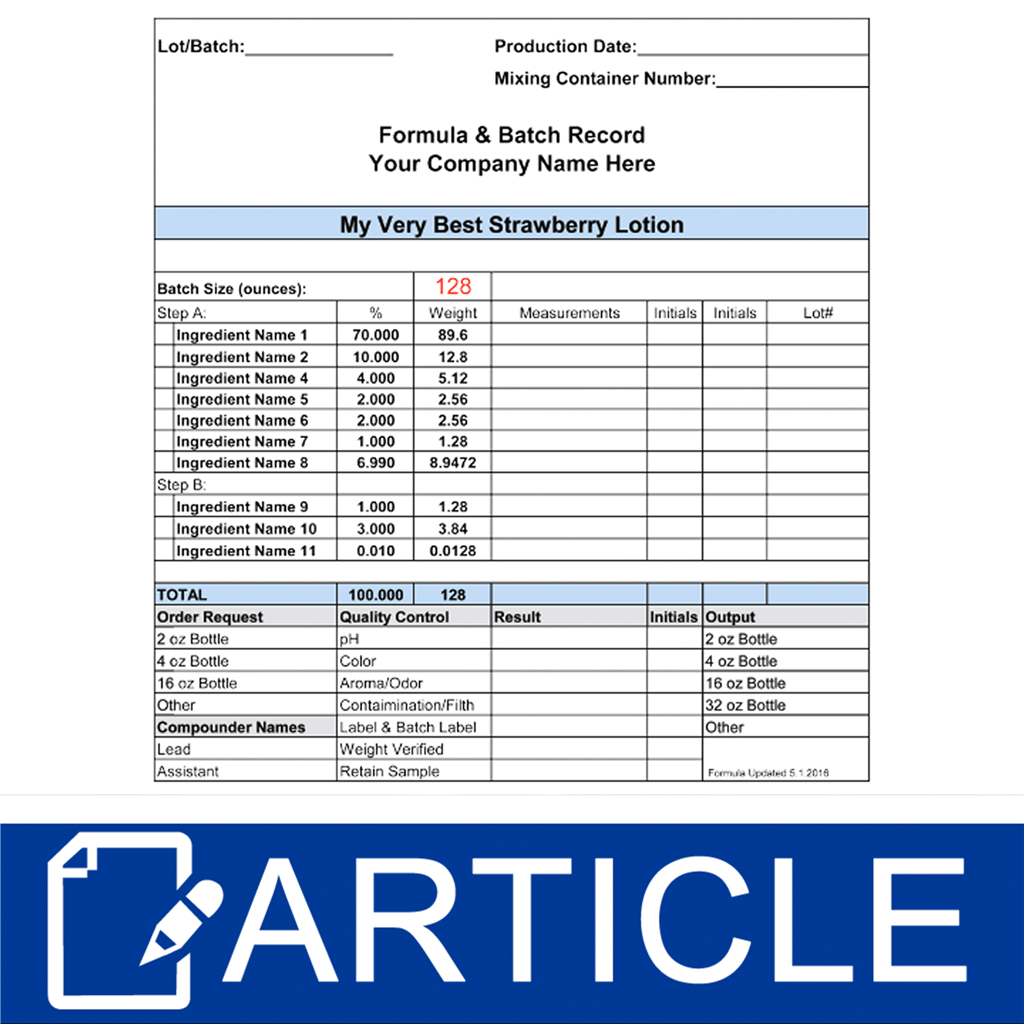
Formula & Batch Records Wholesale Supplies Plus
A Batch Production Record (BPR) is a document that provides a detailed record of the production process for a specific batch of a pharmaceutical product. It includes information on the materials and equipment used, the production steps taken, and the testing and analysis performed throughout the production process.

PHARMACEUTICAL BATCH MANUFACTURING RECORD Sample Download M A N O X B L O G
The batch manufacturing record (BMR) is a document containing the instructions that must be followed when manufacturing medication. It includes information like product name, weight and count of each component in the medication, a list of all processes and procedures to follow, and the expected yield of each batch.

Top Pdf Batch Manufacturing Record Sample Format most complete Forres
The following features can also important for prep batch manufacturing records by the chemical and process manufacturing industry: Step-by-step instructions additionally authentications. BMR software doing a copy a the master formula record, auto-populating instructions how users have lead thru the manufacturing process step by step without.

BMR (Batch Manufacturing Record)
In pharmaceutical manufacturing, MES most often means using electronic batch records (EBR) — a digital record that provides proof of a product's batch record with details on process steps, equipment information, materials and supplies. The MES system is centered in manufacturing operations with connections to other foundational business and.
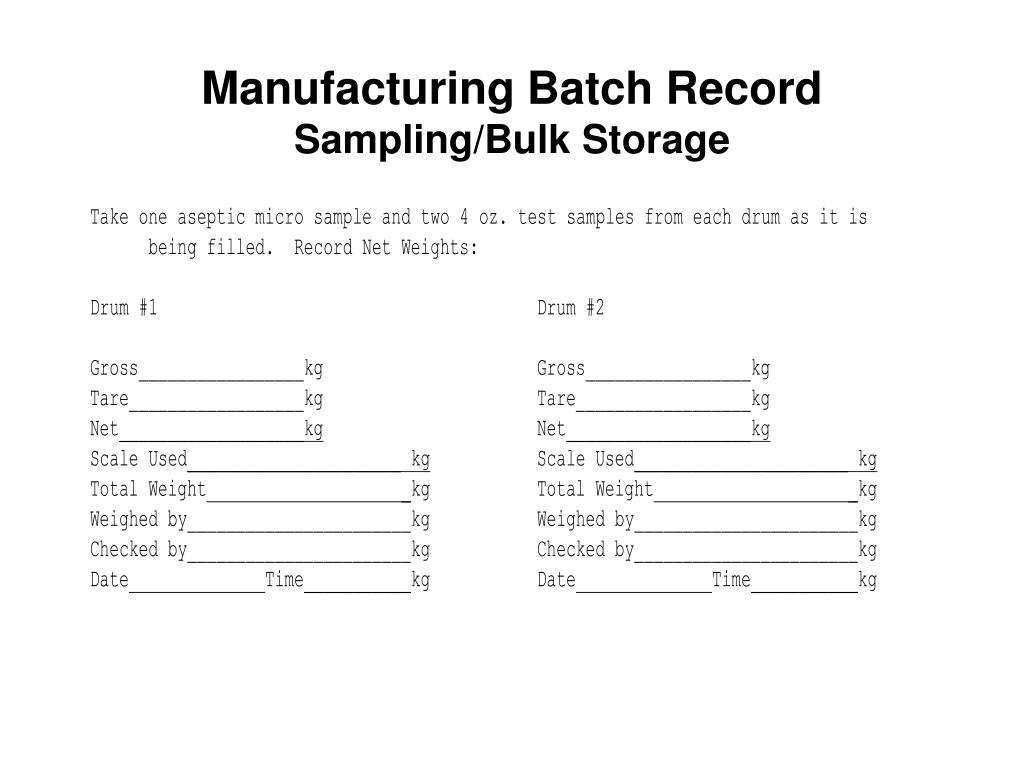
PPT MANUFACTURING DOCUMENTS PowerPoint Presentation, free download ID728573
A batch record is a comprehensive set of documents that outlines all aspects of the Batch manufacturing records (BMRs) Batch production records (BPRs) Master production records (MPRs) This is the most comprehensive type of batch record. It includes all the data associated with the manufacturing process, from raw materials to